Close Packing in Crystalline Solids
Close Packing in Crystalline Solids: Overview
This topic covers concepts, such as, Square Close Packing in Two Dimension, Hexagonal Close Packing in Two Dimension, Close Packing of Lattice Points in Three Dimension & Hexagonal Close Packed Unit Cells etc.
Important Questions on Close Packing in Crystalline Solids
What are the examples of hexagonal close-packed structure?
The pattern in Hexagonal close packing and cubic close packing are same. Is the statement true or false?
The number of atoms in this hcp unit cell is
The number of atoms in the hcp unit cell is_____.
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
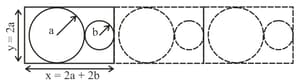
The ratio for which the packing fraction is minimized is closest to:
Which of the following statement is not true about the hexagonal close packing?
In reference to crystal structure, explain the meaning of the coordination number.
How will you distinguish between the following pairs of terms:
Hexagonal close-packing and cubic close packing.
How will you distinguish between the following pairs of terms?
Hexagonal close packing and cubic close packing.
What is the two dimensional coordination number of a molecule in square close packed layer?
Explain with the help of neat diagrams AAAA and ABAB and ABCABC type of three dimensional packings.
The number of atoms present in a hexagonal close packed unit cell is:
Distinguish between the following:
Hexagonal close packing and cubic close packing
In the hexagonal closest packed structure of a metallic lattice, number of the nearest neighbours of a metallic atom is
Which one of the following methods of ordering closed packed sheets of equal sized spheres does not generate close-packed lattice?
The face-centred cubic lattice can be generated by
Hexagonal close-packed arrangement of ions is described as
Which of the following arrangements correctly represents hexagonal and cubic close packed structure respectively?
In a metal oxide, the oxide ions are arranged in hexagonal close packing and metal ions occupy two-third of the octahedral voids. The formula of the oxide is:
What is the formula of a compound of niobium and nitrogen that crystallizes in a hexagonal closest packed array of nitrogen atoms with niobium atoms in half of the tetrahedral holes ?
